MEDIUM
Earn 100
Which one of the following reaction energy diagrams best represents a reaction in the forward direction; that is spontaneous/exothermic and complete in two consecutive steps in which the second step is little more difficult than the first step ?
(a)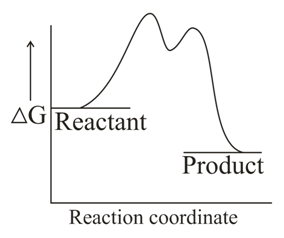

(b)
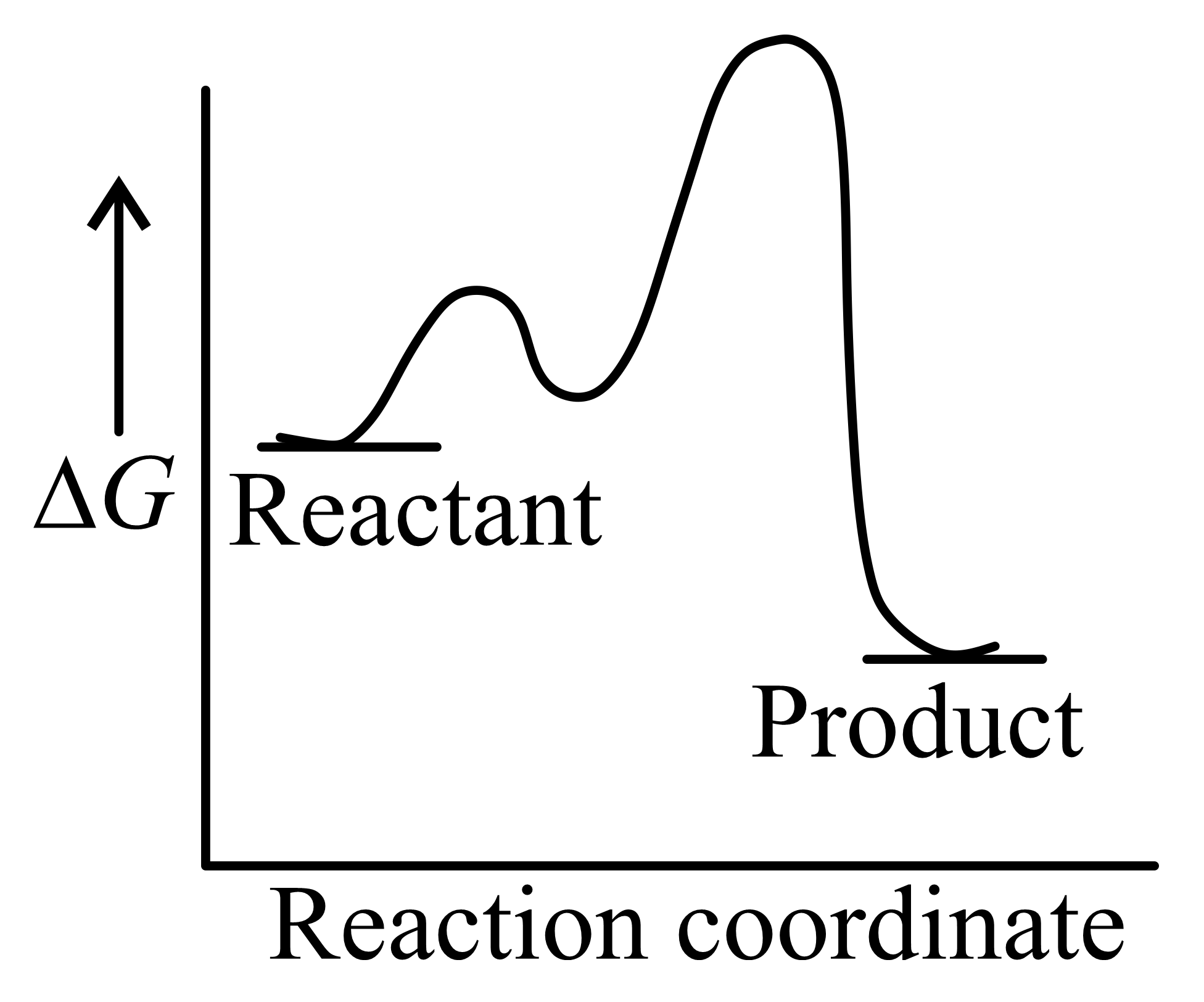
(c)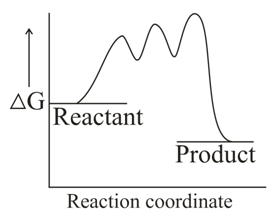

(d)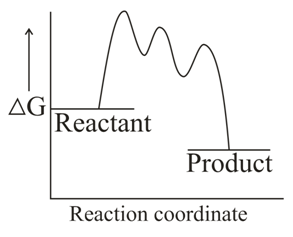

61.01% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
MEDIUM
MEDIUM
Thermal decomposition of occurs as per the equation below :
The correct statement is
MEDIUM
EASY
MEDIUM
In an experiment rate of formation is . Calculate rate of disappearance of ammonia.
EASY
The following results have been obtained during the kinetic studies of reaction:
| Expt | |||
| . | |||
| . | |||
| . |
MEDIUM
EASY
MEDIUM
Given the hypothetical reaction mechanism
and the rate as
| Species formed | Rate of its information |
| per mole of | |
| per mole of | |
| per mole of | |
| per mole of |
The rate determining step is
HARD
HARD
HARD
EASY
EASY
EASY
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

MEDIUM
EASY
EASY

