HARD
Earn 100
Which one of the following reactions involves change from to hybridisation of the central atom?
(a)
(b)
(c)
(d)
(e)
100% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
The percentage of s-character in the hybrid orbitals of nitrogen in and respectively are:
EASY
The number of hybrid orbitals in a molecule of benzene is:
EASY
In molecule, the hybridizations of carbon and oxygen atoms are respectively
EASY
Identify the correct set of molecules with different geometries and central atoms with different hybridisations.
EASY
Which of the following conversions involves change in both shape and hybridisation?
MEDIUM
and are two covalent molecules in which the hybridization of the central atoms is the same, but the shapes are different. What are and ?
MEDIUM
The reaction in which the hybridisation of the underlined atom is affected is
EASY
The state of hybrid orbitals of carbon in and respectively is
EASY
Which of the following pairs of compounds is isoelectronic and isostructural?
EASY
The order of electronegativity of carbon in and hybridized states follows
EASY
The angle in ammonia is while the angle in phosphine is Relative to phosphine, the -character of the lone-pair on ammonia is expected to be
MEDIUM
The incorrect geometry is represented by:
EASY
The hybridisations of and shown in the following compound
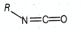
respectively, are
MEDIUM
Among the aliphatic hydrocarbons and the pair having hybrid orbitals to unhybridised orbitals ratio is
EASY
What type of hybridisation takes place in the atom of ?
EASY
The correct order of hybridization used by nitrogen in and is
EASY
In the following molecules,
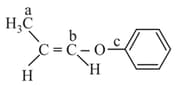
Hybridisation of carbon and respectively are :
EASY
The bond angle H-X-H is the greatest in the compound :
MEDIUM
The hybridisation of atomic orbitals of nitrogen in and , respectively are:
EASY
Consider the molecules and . Which of the given statements is false?

