EASY
Earn 100
Which one of the following relations are used to find saturated vapor pressure at room temperature?
(a)Ideal gas equation
(b)Kelvin-Planck relation
(c)Magnus relation
(d)Clausius-Clapeyron relation
50% studentsanswered this correctly
Important Questions on Calorimetry and Change of State
MEDIUM
EASY
MEDIUM
EASY
EASY
MEDIUM
Assertion (A): The boiling point of water decreases as the altitude increases.
Reason (R): The atmospheric pressure increases with altitude.
EASY
EASY
Vapour that remains in contact with its liquid surface within a closed space is called _____.
(Choose from: saturated vapour/unsaturated vapour)
EASY
EASY
EASY
MEDIUM
EASY
EASY
EASY
The pair of boiling point and compound are given as,
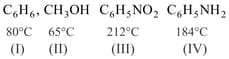
Which will show lowest vapour pressure at room temperature?
MEDIUM
MEDIUM
EASY
EASY
Given, Molar mass of benzene = 78
Molar mass of chlorobenzene = 112.5
| Temperature (0oC) |
Vapour pressure of benzene (torr) |
Vapour pressure of chlorobenznee (torr) |
|---|---|---|
| 80 | 750 | 120 |
| 90 | 1000 | 200 |
| 100 | 1350 | 300 |
| 110 | 1800 | 400 |
| 120 | 2200 | 540 |
MEDIUM

