EASY
Earn 100
Which one of the following salt give green coloured flame when the salt is tested by wire
(a)Barium salt
(b)Calcium salt
(c)Borate
(d)Lead salt
50% studentsanswered this correctly
Important Questions on Theoretical Principles of Experimental Chemistry
HARD
Distinguish between the following pair of compounds using a reagent as a chemical test:
- Magnesium chloride and magnesium nitrate solution.
MEDIUM
A chloride salt solution acidified with dil. gives a curdy white precipitate, , on addition of . [A] on treatment with gives a clear solution, B.
MEDIUM
State one relevant observation for of the following:
Anhydrous calcium chloride is exposed to air for some time.
MEDIUM
The green colour produced in the borax bead test of a chromium(III) salt is due to -
EASY
When treated with conc. yields gas which further reacts with to generate a white solid reacts with dil. to produce the same gas the solid is
MEDIUM
Which combines with to form brown complex?
MEDIUM
When a solution of mixture having two inorganic salts was treated with freshly prepared ferrous sulphate in acidic medium, a dark brown ring was formed whereas on treatment with neutral , it gave deep red colour which disappeared on boiling and a brown red ppt was formed. The mixture contains
MEDIUM
Consider the following reactions:
side products
side products
side products
The sum of the total number of atoms in one molecule each of and is ________
EASY
Match List-I with List-II and select the correct answer using the codes given below.
| List I | List II | ||
| A. | Rinmann's green | 1. | |
| B. | Verdigris | 2. | |
| C. | Corrosive sublimate | 3. | |
| D. | Lithopone | 4. |
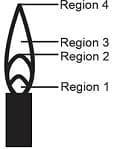
EASY
The hottest region of Bunsen flame shown in the figure below is:
MEDIUM
When a substance 'A' reacts with water, it produces a combustible gas 'B' and a solution of substance 'C' in water. While another substance 'D' reacts with solution of 'C' to produce the same gas B on warming while 'D' can produce gas 'B' on reaction with dilute at room temperature. 'A' imparts a deep golden - yellow colour to a smokeless flame on Bunsen burner. Identify 'A', 'B', 'C' and 'D' respectively are
MEDIUM
The dark red colour of bombs in fireworks is due to the presence of
MEDIUM
On mixing two colourless gases, a deep brown colour is observed. The gases are
EASY
A salt gives bright red colour to the flame. This colour indicates the presence of
MEDIUM
A pale yellow precipitate and a gas with pungent odour are formed on warming dilute hydrochloric acid with an aqueous solution containing :
MEDIUM
Which of the following salt gives white precipitate with solution and dil. solution and gives green flame test
HARD
Identify (W) to (Z) :
EASY
A colourless gas with the smell of rotten fish is
HARD
Statement I : Borax bead test is not suitable for Al(III).
Statement II : Al2O3 is insoluble in water.
MEDIUM
Which of the following sulphate is insoluble in water

