EASY
JEE Main
IMPORTANT
Earn 100
Which one of the following statements is FALSE for hydrophilic sols?
(a)These sols are reversible in nature.
(b)Their viscosity is of the order of that of .
(c)The sols cannot be easily coagulated.
(d)They do not require electrolytes for stability.
50% studentsanswered this correctly

Important Questions on Surface Chemistry
EASY
JEE Main
IMPORTANT
In Freundlich adsorption isotherm at moderate pressure, the extent of adsorption is directly proportional to . The value of is :
MEDIUM
JEE Main
IMPORTANT
Most suitable salt which can be used for efficient clotting of blood will be?
MEDIUM
JEE Main
IMPORTANT
In Freundlich adsorption isotherm, slope of line is:
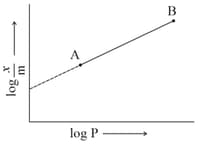
MEDIUM
JEE Main
IMPORTANT
When silver nitrate solution is added to potassium iodide solution then the sol produced is:
MEDIUM
JEE Main
IMPORTANT
For coagulation of 50 mL of a sol, 10 mL of 0.5M Cl- ion solution is required. What is the coagulating value of ion solution (Nearest integer)
MEDIUM
JEE Main
IMPORTANT
The conditions given below are in the context of observing Tyndall effect in colloidal solutions:
(A) The diameter of the colloidal particles is comparable to the wavelength of light used.
(B) The diameter of the colloidal particles is much smaller than the wavelength of light used.
(C) The diameter of the colloidal particles is much larger than the wavelength of light used.
(D) The refractive indices of the dispersed phase and the dispersion medium are comparable.
(E) The dispersed phase has a very different refractive index from the dispersion medium. Choose the most appropriate conditions from the options given below:
MEDIUM
JEE Main
IMPORTANT
Amongst the following statements regarding adsorption, those that are valid are:
(a) becomes less negative as adsorption proceeds.
(b) On a given adsorbent, ammonia is adsorbed more than nitrogen gas.
(c) On adsorption, the residual force acting along the surface of the adsorbent increases
(d) With the increase in temperature, the equilibrium concentration of adsorbate increases.
MEDIUM
JEE Main
IMPORTANT
Which of the following is used for the preparation of colloids ?
