EASY
Earn 100
Which one of the following statements is true, in respect of the usual quantities represented by and
(a) and are path dependent
(b) and are path dependent
(c) does not depends upon path.
(d) does not depends upon path
50% studentsanswered this correctly
Important Questions on Thermodynamics
EASY
EASY
A gas at state changes to state through path and shown in figure. The change in internal energy is and , respectively. Then
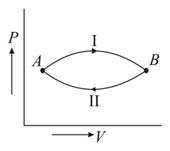
EASY
A given quantity of gas is taken from the state to the state reversibly by two paths, directly and as shown in the figure below:
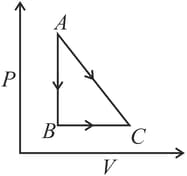
During the process , work done by the gas is and heat absorbed is . If during the process , the work done by the gas is , the heat absorbed is
EASY
[Take ]

EASY
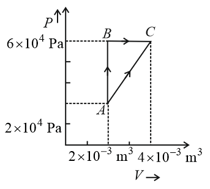
In process AB, of heat is added to the system and in process BC, of heat is added to the system. The heat absorbed by the system in the process AC will be:
EASY
EASY
EASY
(use )
EASY
When a system is taken from state to state along the path , it is found that and . Along the path , . along the path is,
EASY
EASY
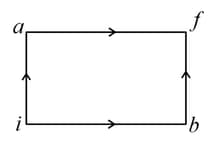
An ideal gas is taken from state- to state- through optional path and as shown in the diagram. Let and represent the heat supplied, work done and change internal energy respectively, then
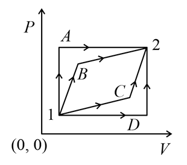
MEDIUM
EASY
EASY
EASY
EASY
EASY
Match the following?
| Column - I | Column - II | ||
| (a) | Isothermal process | (i) | No heat exchange |
| (b) | Adiabatic process | (ii) | Constant temperature |
| (c) | Isochoric process | (iii) | Constant pressure |
| (d) | Isobaric process | (iv) | Constant volume |
EASY
EASY
EASY

