MEDIUM
Earn 100
Which port in India handles the highest volume of cargo traffic?
(a)Mumbai Port
(b)Chennai Port
(c)Kandla Port
(d)Visakhapatnam Port
50% studentsanswered this correctly
Important Questions on Solutions
MEDIUM
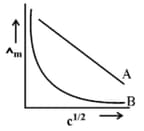
In the plot of molar conductivity vs square root of concentration , following curves are obtained for two electrolytes A and B :
Answer the following :
Predict the nature of electrolytes A and B.
MEDIUM
MEDIUM
MEDIUM
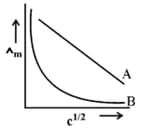
In the plot of molar conductivity vs square root of concentration , following curves are obtained for two electrolytes A and B :
Answer the following :
What happens on extrapolation of to concentration approaching zero for electrolytes A and B ?
EASY
MEDIUM
is , then the cell constant of the conductivity cell is____
MEDIUM
EASY
: Conductivity always increases with decreases in the concentration of electrolyte.
: Molar conductivity always increases with decreases in the concentration of electrolyte.
The correct option among the following
EASY
MEDIUM
EASY
HARD
MEDIUM
Determination of the molar mass of acetic acid in benzene using freezing point depression is affected by:
EASY
EASY
EASY