MEDIUM
Earn 100
Which principle is based on the premise that a business will continue to operate indefinitely?
(a)Going Concern Principle
(b)Consistency Principle
(c)Disclosure Principle
(d)Materiality Principle
50% studentsanswered this correctly
Important Questions on Waves
MEDIUM
EASY
EASY
EASY
EASY
The pressure (P) and density of a diatomic gas changes from to . What is the value of if
EASY
EASY
EASY
EASY
EASY
EASY
EASY
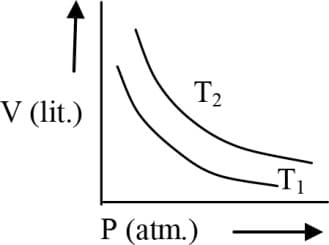
EASY
EASY
One mole of an ideal gas at is expanded isothermally from an initial volume of litre to litre. for this process is:
EASY
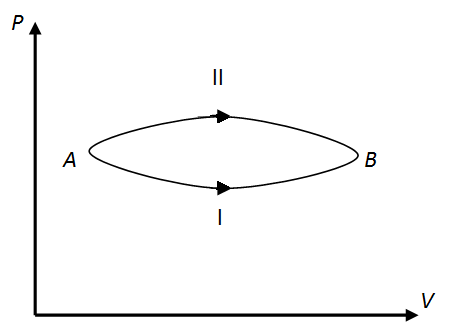
EASY
EASY
MEDIUM
EASY
MEDIUM

