MEDIUM
Earn 100
Which process involves the expansion and contraction of rocks due to temperature changes?
(a)Exfoliation
(b)Frost wedging
(c)Oxidation
(d)Hydration
50% studentsanswered this correctly
Important Questions on Thermodynamics
EASY
MEDIUM
EASY
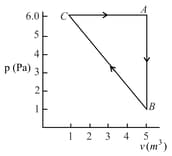
For the given cyclic process as shown for a gas, the work done is:
EASY
EASY
EASY
MEDIUM
EASY
EASY
EASY
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
EASY
EASY
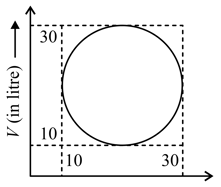
Heat energy absorbed by a system in going through a cyclic process shown in diagram.
MEDIUM
EASY

