EASY
Earn 100
Which reagent is used in the ammonolysis of alcohol?
Important Questions on Organic Compounds Containing Nitrogen
EASY
HARD
HARD
Starting from propanoic acid, the following reactions were carried out
Propanoic acid
What is the compound ?
MEDIUM
MEDIUM
EASY
Identify in the following reaction
EASY
MEDIUM
MEDIUM
EASY
HARD
EASY
MEDIUM
MEDIUM
What are and in the following reactions?
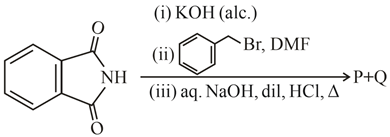
MEDIUM
MEDIUM
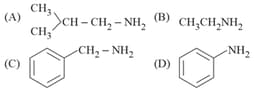
EASY
EASY
EASY
MEDIUM

