MEDIUM
Earn 100
Which situation is not characteristic of the collision theory model of the reaction ?
(a)The electron density around A, B, and C must change
(b)The orientation of the two species must be correct
(c)As A approaches BC, a strong attraction between any filled shells of electrons will develop between the two species
(d)The kinetic energy of the molecules may be the only source of energy for attaining the activation energy. The collisions of A and BC that result in a reaction are a very small fraction of the total number of collisions
100% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
EASY
MEDIUM
MEDIUM
(Assume Activation energy and pre-exponential factor are independent of temperature; )
MEDIUM
MEDIUM
MEDIUM
[Gas constant, ]
MEDIUM
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

MEDIUM
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is:
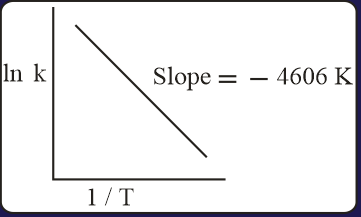
MEDIUM
The Arrhenius plots of two reactions, I and II are shown graphically-
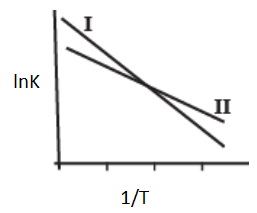
The graph suggests that-
EASY
EASY
EASY
MEDIUM
A sample of milk splits after at and after at when the population of Iactobacillus acidophilus in it doubles. The activation energy (in ) for this process is closest to ______________.
MEDIUM
MEDIUM
HARD
The activation energy of the backward reaction exceeds that of the forward reaction by (in ). If the pre-exponential factor of the forward reaction is times that of the reverse reaction, the absolute value of for the reaction at is ____.
(Given; and is the Gibbs energy)
EASY
EASY

