Which statements about substitution reactions are correct?
I. The reaction between sodium hydroxide and 1-chloropentane predominantly follows an mechanism.
II. The reaction between sodium hydroxide and 2-chloro-2-methylbutane predominantly follows an mechanism.
III. The reaction of sodium hydroxide with 1-chloropentane occurs at a slower rate than with 1-bromopentane.

Important Questions on Organic Chemistry [AHL]
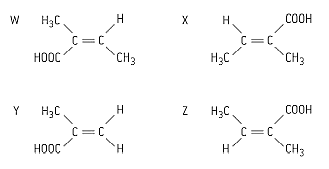
Which two molecules in given figure are cis–trans isomers of each other?
Halogenoalkanes can undergo substitution reactions with potassium hydroxide solution.
State an equation for the reaction of with .
Substitution reactions may occur by either of two mechanisms namely or . Outline the meaning of the term .
Predict the mechanism expected for the reaction of the following halogenoalkanes with aqueous .
- 1-chlorobutane to form butan-1-ol
- 2-chloro-2-methylpropane to form 2-methylpropan-2-ol.
Explain the mechanism of each of the following reactions using curly arrows to represent the movement of electron pairs.
- 1-chlorobutane to form butan-1-ol
- 2-chloro-2-methylpropane to form 2-methylpropan-2-ol.
There are several structural isomers with the molecular formula .
Deduce the name of one of the isomers which can exist as enantiomers and draw three-dimensional representations of its two enantiomers.
