EASY
Earn 100
Which statements of the following is incorrect.
(a)Molecularity of a reaction is always a whole number
(b)Order and molecularity of a reaction need not be same
(c)Order of a reaction may be zero
(d)Order of a reaction depends upon the mechanism of the reaction
50% studentsanswered this correctly
Important Questions on Chemical Kinetics
MEDIUM
EASY
The kinetic order for the following reaction is:
EASY
HARD
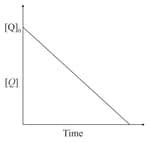
the time taken for reaction of is twice the time taken for reaction of . The concentration of varies with reaction time as shown in the figure.The overall order of the reaction is:
EASY
EASY
MEDIUM
EASY
EASY
MEDIUM
EASY
MEDIUM
For the chemical reaction
, the reaction proceeds as follows
(Fast)
, (Slow)
the rate law expression should be given as
MEDIUM
EASY
EASY
EASY
MEDIUM
EASY
EASY
EASY

