Which states of matter are being described here? Complete the table.
The first example has been done for you.
Description
State or states
Occupies a fixed volume
Solid, liquid
Evaporates to become a gas
Takes the shape of its container
Has a fixed volume
May become a liquid when its temperature changes
The first example has been done for you.

Important Questions on The Kinetic Model of Matter
Label each arrow in the diagram below to show the name of the change of state.
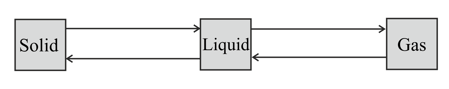
State the temperature at which water becomes ice.
Describe and explain the changes in the molecular structure of water, in the form of ice as it is heated until the water has become steam. Consider the arrangement of molecules.
Describe and explain the changes in the molecular structure of water, in the form of ice as it is heated until the water has become steam. Consider the movement of molecules.
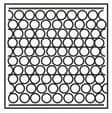
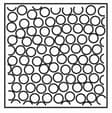
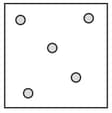
Complete the table. In the first row, draw sketches of the three states of matter in terms of the arrangement of the particles and their movement. Then describe the three states, referring to the three criteria in the first column.
| State | Solid | Liquid | Gas |
 |
 |
 |
|
| How close are particles to their neighbours? | |||
| How do the particles move? | |||
| Strength of forces between particles (strong/weak/zero) |
What is the word we use to describe the process of a liquid becoming a gas?
