EASY
Earn 100
Who proposed the law of octaves?
(a)Newland
(b)Dobereiner
(c)Henry Moseley
(d)Mendeleev
50% studentsanswered this correctly
Important Questions on The Periodic Table
MEDIUM
EASY
HARD
MEDIUM
Write a short note on the position of isotopes in the Mendeleev’s and the modern periodic table.
MEDIUM
HARD
EASY
HARD
EASY
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
(a) Name the elements which took over their positions.
(b) Name the groups to which these elements belong and also give their electronic configuration.
(c) Predict the valencies of these elements.
MEDIUM
MEDIUM
Write a short note on Mendeleev’s periodic law.
MEDIUM
EASY
HARD
Rearrange the columns and to match with the column .
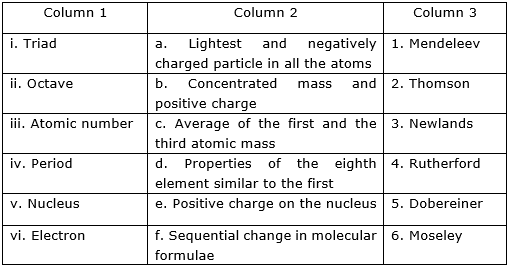
MEDIUM

