EASY
Earn 100
Why is non-polar and is polar although and both molecules are covalent?
(a)In uncombined state boron is metal and nitrogen is gas
(b) bond has no dipole moment whereas bond has dipole moment
(c)The size of boron atom is smaller than nitrogen
(d) is planar whereas is pyramidal
50% studentsanswered this correctly
Important Questions on Chemical Bonding and Molecular Structure
EASY
MEDIUM
EASY
EASY
HARD
MEDIUM
HARD
HARD
EASY
EASY
EASY
HARD
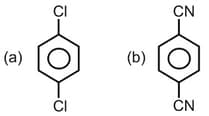
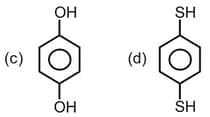
EASY
EASY
EASY
HARD
EASY
MEDIUM
EASY
EASY

