EASY
Earn 100
Why do some elements form diatomic or triatomic molecules?
Important Questions on Atoms and Molecules
EASY
EASY
In the following circuit diagram, the current through the battery is
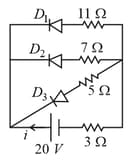
EASY
Match the following
List - I List-II
A) Metallic solid I) Carbon tetrachloride
B) Covalent solid II) Calcium fluoride
C) Molecular solid III) Copper
D) Ionic solid IV) Silicon carbide
V) Glass
The correct answer is
MEDIUM
EASY
EASY
EASY
EASY
type of semi-conductor material
amount of doping
temperature
Which one of the following is correct?
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY
This solid is most likely to be a / an :
HARD
The cubic unit cell structure of a compound containing cation and anion is shown below. When compared to the anion, the cation has smaller ionic radius. Choose the correct statement(s).
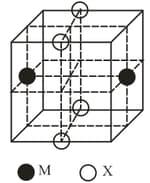
EASY
HARD
Remove all the anions , except the central one.
Replace all the face centered cations , by anions .
Remove all the corner cations .
Replace the central anion , with cation .
The value of in is _____.
