HARD
Earn 100
Why ethylene glycol, have a much lower equilibrium vapor pressure than octane?
Important Questions on States of Matter
HARD

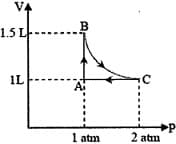
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
HARD
HARD

The correct option(s) is (are)
EASY
EASY
(Latent heat of ice is and )
MEDIUM
MEDIUM
The combination of plots which does not represent isothermal expansion of an ideal gas is




EASY
HARD
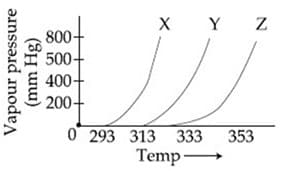
The following inferences are made:
has higher intermolecular interactions compared to
has lower intermolecular interactions compared to
has lower intermolecular interactions compared to
The correct inferences is/are:
EASY
EASY
MEDIUM
[ Specific heat of water Latent heat of water ]
MEDIUM
[Heat of fusion of ice ; Specific heat of water ]
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
EASY
MEDIUM
MEDIUM
MEDIUM

MEDIUM
EASY

