MEDIUM
12th Andhra Pradesh Board
IMPORTANT
Earn 100
Why is alternating current used for measuring the resistance of an electrolytic solution?

Important Questions on Electrochemistry and Chemical Kinetics
MEDIUM
12th Andhra Pradesh Board
IMPORTANT
Solutions of two electrolytes and are diluted. The of increases times while that of increases times. Which of the two is a strong electrolyte? Justify your answer.
MEDIUM
12th Andhra Pradesh Board
IMPORTANT
Why on dilution the of increases drastically, while that of increases gradually?
EASY
12th Andhra Pradesh Board
IMPORTANT
In an aqueous solution how does specific conductivity of electrolytes change with addition of water?
MEDIUM
12th Andhra Pradesh Board
IMPORTANT
Using the standard electrode potentials, predict if the reaction between the following is feasible:
and
, .
MEDIUM
12th Andhra Pradesh Board
IMPORTANT
The standard EMF of the cell : is volt. The standard electrode potential (reduction potential) of copper electrode is volt. Calculate the standard electrode potential of nickel electrode.
MEDIUM
12th Andhra Pradesh Board
IMPORTANT
Give appropriate scientific reasons for the following statement:
During electrolysis of molten lead bromide, graphite anode is preferred to other electrodes.
MEDIUM
12th Andhra Pradesh Board
IMPORTANT
The following is a sketch of an electrolytic cell used in the extraction of aluminium:
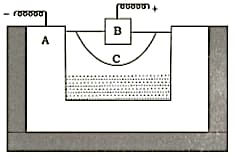
(i) What is the substance of which the electrodes and are made?
(ii) At which electrode (A or B) is the aluminium formed?
(iii) What are the two aluminium compounds in the electrolyte C?
(iv) Why is it necessary for electrode B to be continuously replaced?
MEDIUM
12th Andhra Pradesh Board
IMPORTANT
If and are standard oxidation potentials for and then
The value of is?
