EASY
JEE Advanced
IMPORTANT
Earn 100
Why is the second ionisation energy of lithium much greater than that of beryllium?

Important Questions on Periodic Classification of Elements and General Inorganic Chemistry
HARD
JEE Advanced
IMPORTANT
The energy needed for is . If the first ionisation energy of is , calculate the second ionisation energy for . Given for .
MEDIUM
JEE Advanced
IMPORTANT
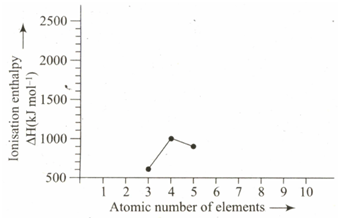
The ionisation enthalpies of elements of the second period are given below:
Ionisation enthalpy
Match the correct enthalpy with the elements and complete the graph given in the following figure. Also write the symbols of elements with their atomic numbers.
EASY
JEE Advanced
IMPORTANT
The and of an element are , respectively. The element is likely to be:
EASY
JEE Advanced
IMPORTANT
Consider the following changes:
(a) Sublimation energy of
(b) Sublimation of
(c) of
(d) of
(e) of
The enthalpy change for the reaction could be calculated from the energy value associated with:
EASY
JEE Advanced
IMPORTANT
Successive ionisation energies of an element are given below (in ):
Electronic configuration of the element is:
EASY
JEE Advanced
IMPORTANT
Which systematic diagram is correct about the ionisation energy of coinage metals?
MEDIUM
JEE Advanced
IMPORTANT
of Avogadro's number of atoms of an element absorb energy . The ionisation energy of the element is:
EASY
JEE Advanced
IMPORTANT
A sudden large jump between the values of the second and the third ionisation energies of an element would be associated with the electronic configuration:
