MEDIUM
Earn 100
Why work done in a free expansion of ideal gas is zero?
Important Questions on Thermodynamics
EASY
EASY
Under the isothermal condition, a gas at expands from to against a constant external pressure of bar. The work done by the gas is
(Given that bar)
MEDIUM
EASY
EASY
HARD
EASY
EASY
MEDIUM
EASY
MEDIUM
HARD
(Given,
EASY
EASY
MEDIUM
[ Use ]
MEDIUM
(Round off to the Nearest Integer)
[Use :
[Assume volume of is much smaller than volume of . Assume treated as an ideal gas]
HARD
At of iron reacts with to form . The evolved hydrogen gas expands against a constant pressure of . The work done by the gas during this expansion is -_______ .
(Round off to the Nearest Integer)
[Given : . Assume, hydrogen is an ideal gas]
[Atomic mass off Fe is ]
MEDIUM
The magnitude of work done by a gas that undergoes a reversible expansion along the path shown in the figure is _________.
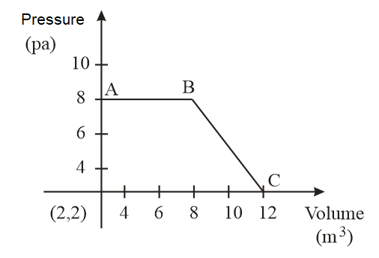
HARD
MEDIUM

