HARD
JEE Advanced
IMPORTANT
Earn 100
With respect to the compounds choose the correct statement(s).
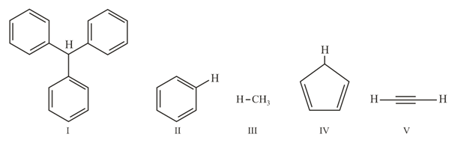

(a)The acidity of compound is due to delocalization in the conjugate base.
(b)The conjugate base of compound is aromatic.
(c)Compound becomes more acidic, when it has a substituent.
(d)The acidity of compounds follows the order .
20.88% studentsanswered this correctly

Important Questions on Organic Chemistry- Some Basic Principles and Techniques
EASY
JEE Advanced
IMPORTANT
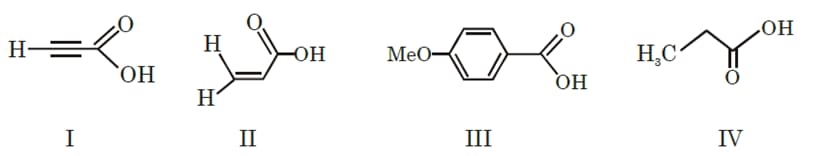
The correct order of acid strength of the following carboxylic acids is -
MEDIUM
JEE Advanced
IMPORTANT
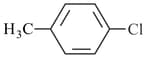
The IUPAC name(s) of the following compound is(are)
MEDIUM
JEE Advanced
IMPORTANT
The compound that does NOT liberate CO2, on treatment with aqueous sodium bicarbonate solution, is
HARD
JEE Advanced
IMPORTANT
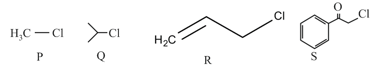
KI in acetone, undergoes SN2 reaction with each of P, Q, R and S. The rates of the reaction vary as
MEDIUM
JEE Advanced
IMPORTANT
The hyperconjugative stabilities of tert-butyl cation and 2-butene, respectively, due to
