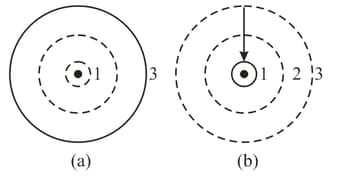
Within the framework of the "classical" Bohr theory, an excited atom is an atom one electron of which moves along an orbit that is farther from the nucleus than in the ground state (Figure (a)). When the atom goes over to its ground state (Figure (b)), the atom emits a photon. In the literature, especially popular-science literature, the common way to describe this process is to say that mass has transformed into energy. Is this' actually the case?


Important Questions on Atomic and Nuclear Physics
A proton that has flown over a great distance hits. a proton that is at rest. The impact parameter is zero, that is, the velocity of the incident proton is directed along the straight line connecting the centres of the protons. The mass of the proton is known, , and the initial velocity of the incident proton is . How close will the incidence proton get to the fixed proton?
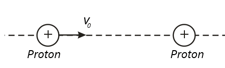
Suppose that the energy required to ionize a hydrogen atom is . Must the electron, the hydrogen ion, and the helium ion have the same initial kinetic energies for the hydrogen atom to become ionized?
The system of quantum levels of an atom is assumed to be like the one depicted in the figure. How will each of the energy components of the electron (the kinetic energy and the potential energy) vary if the electron moves from a lower level to a higher level?
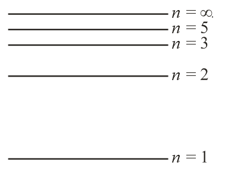
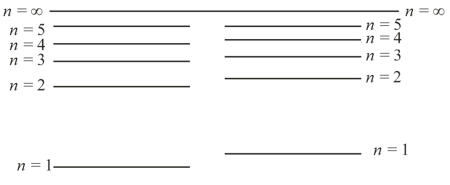
The quantum levels of atoms of hydrogen and deuterium are only approximately the same (the difference between the two systems of levels is exaggerated in the figure). Which system of levels belongs to which atom? What is the reason for this discrepancy?
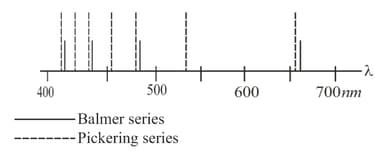
Every other spectral line in one of the spectral series of an ionized helium atom (the Pickering series) closely resembles a line in the Balmer series for hydrogen. What is the principal quantum number of the level to which the electrons transfer when these lines are emitted? Why don't the lines coincide exactly? What is the meaning of the lines that lie in between the lines of the Balmer series?
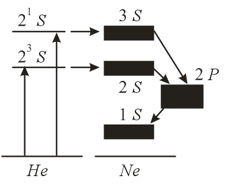
In a laser, the helium atoms are excited from the ground state to two sublevels, and , interact with atoms, and give off their energy to atoms, with the result that the latter are transferred to the and levels.
The Ne atoms in these states emit radiation and go over to the level. In the figure, the and levels, each consisting of four sublevels, and the level, which consists of ten sublevels, are depicted by broad black bands. In addition to the above-mentioned transitions, a transition from the state to the level is possible, but we do not show this transition in the figure.
From the state, atoms go over to the state, and then gradually return to their ground state. Why don't He atoms emit radiation during transitions from the and states directly to the ground state? What must be the relationship between the lifetimes of He atoms in states , and for continuous generation of radiation to be possible? It has been established that of the two transitions, and , one is accompanied by radiation in the visible spectrum and the other, in the IR spectrum. Which transition corresponds to which spectrum?