MEDIUM
Earn 100
Work Done in Thermodynamical Process, Law, Internal Energy & Heat Capacity. The internal energy when a system goes from state A to B is 40 kJ/mol. If the system goes from A to B by a reversible path and returns to state A by an irreversible path. What would be the net change in internal energy?
(a)40 kJ
(b)> 40 kJ
(c)< 40 kJ
(d)Zero
50% studentsanswered this correctly
Important Questions on Thermodynamics
EASY
EASY
MEDIUM
MEDIUM
(R = 8.314 J/mol K) (ln7.5 = 2.01)
EASY
MEDIUM
HARD
The specific heat of a certain substance is . Assuming ideal solution behavior, the energy required (in ) to heat of molal of its aqueous solution from to is closest to :
[Given: molar mass of the substance ; specific heat of water ]
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
of nitrous oxide gas is cooled at a constant pressure of atm from to causing the compression of the gas from to . The change in internal energy of the process, is . The value of is _____.
[nearest integer]
(Given: atomic mass of and of . Molar heat capacity of is )
EASY
EASY
EASY
HARD
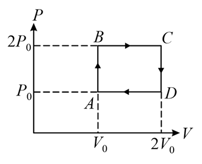
The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:

