EASY
NEET
IMPORTANT
Earn 100
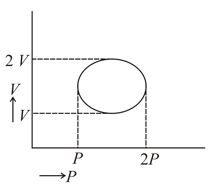
Work done in isochoric process is:-
(a)
(b)
(c)
(d)None of these
70.59% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
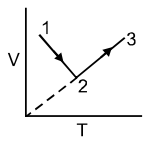
Incorrect statement is:
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
thermodynamic system goes in cyclic reversible process as represented in the following diagram:
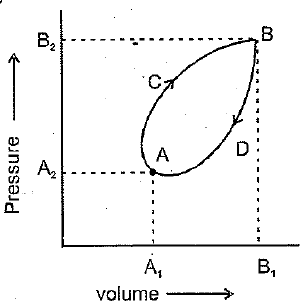
The net work done during the complete cycle is given by the area :
EASY
NEET
IMPORTANT
MEDIUM
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
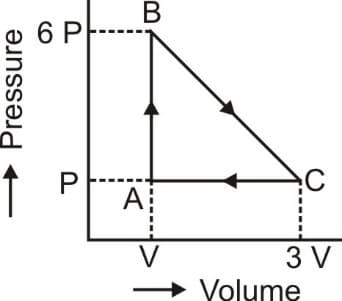
In cyclic process, the magnitude of work done is:-