EASY
AP EAPCET
IMPORTANT
Earn 100
Work done on (or) by a thermodynamic system is zero in which of the following processes?
68.97% studentsanswered this correctly
Important Questions on Thermodynamics
HARD
AP EAPCET
IMPORTANT
A gas consisting of rigid molecules expands adiabatically such that r.m.s. speed of its molecules becomes half. The ratio of final and initial volumes of the gas is (ratio of specific heat capacities of the gas is )
HARD
AP EAPCET
IMPORTANT
Certain quantity of heat is supplied to a monatomic ideal gas which expands at constant pressure. The percentage of heat that is used to do work by the gas is
EASY
AP EAPCET
IMPORTANT
When the state of a gas is adiabatically changed from an equilibrium state to another equilibrium state the amount of work done on the system is If the gas is taken from state to via a process in which the net heat absorbed by the system is the net work done by the system in joule
MEDIUM
AP EAPCET
IMPORTANT
If be the universal gas constant then, the amount of heat required to raise the temperature of moles of monoatomic gas under isobaric condition from to will be:
EASY
AP EAPCET
IMPORTANT
Figure below shows a cyclic process . If be the heat supplied to the system. be the change in internal energy and be the work done by the system, then which of the following relation is correct ?
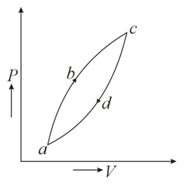
MEDIUM
AP EAPCET
IMPORTANT
What amount of heat is to be supplied to of Nitrogen at room temperature, to rise its temperature by at a constant pressure? and molecular weight of nitrogen is
MEDIUM
AP EAPCET
IMPORTANT
Two moles of helium gas are taken along the path (as shown). The work done by the gas is _______
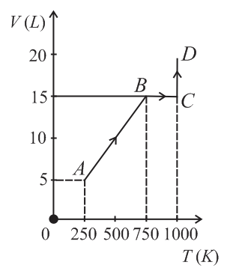
EASY
AP EAPCET
IMPORTANT
Assume that you have an ideal gas for which initially at atm pressure. When the gas is compressed to half its original volume, then the final pressure, if the compression is isothermal, is __________
