EASY
Earn 100
Write Arrhenius equation showing the effect of temperature on the reaction rate. What do different symbols signify? How does it help in the calculation of activation energy of a reaction?
Important Questions on Chemical Kinetics
EASY
HARD
Consider the given plots for a reaction obeying Arrhenius equation (and are rate constant and activation energy, respectively )
(I)

(II)

MEDIUM
The Arrhenius plots of two reactions, I and II are shown graphically-
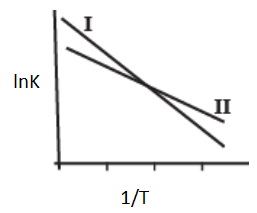
The graph suggests that-
MEDIUM
A sample of milk splits after at and after at when the population of Iactobacillus acidophilus in it doubles. The activation energy (in ) for this process is closest to ______________.
MEDIUM
[Gas constant, ]
HARD
MEDIUM
(Assume Activation energy and pre-exponential factor are independent of temperature; )
MEDIUM
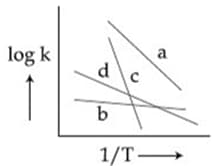
MEDIUM
MEDIUM
MEDIUM
HARD
MEDIUM
HARD
The activation energy of the backward reaction exceeds that of the forward reaction by (in ). If the pre-exponential factor of the forward reaction is times that of the reverse reaction, the absolute value of for the reaction at is ____.
(Given; and is the Gibbs energy)
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
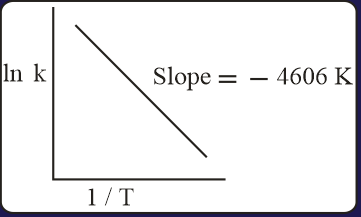
For a reaction, consider the plot of versus given in the figure. If the rate constant of this reaction at is , then the rate constant at is: