MEDIUM
Earn 100
Write about the inspiration behind the law of constant proportions.
Important Questions on Reacting Masses and Chemical Equations
MEDIUM
The reaction of the burning of carbon in oxygen is represented by equation
When 9.0 g of solid carbon is burnt in 16.0 g of oxygen gas the mass of carbon dioxide gas formed would be:
(Note: Atomic mass of C-12,o u, O=16.0 u)
MEDIUM
Two samples A and B of a pure substance containing elements Y and Z are obtained from two different sources. of sample A contains of Z. Sample B is made of of Y by weight. This is an illustration of which of the following laws?
MEDIUM
Magnesium and oxygen combine in the ratio of by mass to form magnesium oxide. How much mass of oxygen is required to react completely with g of magnesium?
EASY
What mass of oxygen, would be required to react completely with 3 g of hydrogen gas?
EASY
Atomic theory was proposed by:
MEDIUM
Select from the following figure(s) that correctly represent(s) the experimental set-up for the verification of conservation of mass in a chemical reaction.
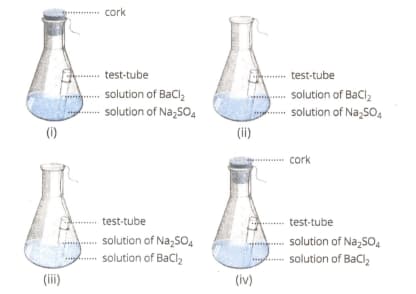
EASY
Law of constant proportion is also known as?
MEDIUM
Name one Indian and one foreign ancient thinker, who proposed the atomic nature of matter.
MEDIUM
Which postulate of Dalton's atomic theory is the result of the law of conservation of mass?
EASY
The indivisibility of atom was proposed by
EASY
According to Dalton's atomic theory, the smallest particle of matter is___________.
EASY
Theory of constant composition was proposed by:
EASY
In ammonia, nitrogen and hydrogen are always present in the ratio?
MEDIUM
Which postulate of Dalton's atomic theory can explain:
(a) the law of conservation of mass,
(b) the law of definite proportion?
EASY
Which of the following is a postulate of Dalton's atomic theory?
EASY
Law of conservation of mass was attributed by:
EASY
The law of constant proportion was stated by Proust as?
EASY
Which postulate of Dalton's atomic theory can explain the law of definite proportions?

