HARD
Earn 100
Write all the possible structural isomers of alcohol having molecular formula . Give their IUPAC names. Classify them as primary, secondary and tertiary alcohols. Identify optically active alcohols amongst them.
Important Questions on Alcohols, Phenols and Ethers
EASY
Write the IUPAC name of the following:
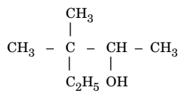
MEDIUM
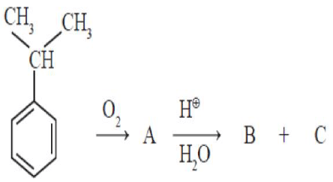
HARD
EASY
EASY
Which of the following is/are aromatic alcohol?
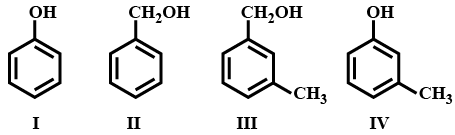
EASY
EASY
MEDIUM
EASY
EASY
MEDIUM
Write the IUPAC name of
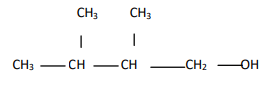
EASY
MEDIUM
HARD
Draw the structural formula for the following:
- methanol
MEDIUM
MEDIUM
EASY
EASY
EASY
MEDIUM
