HARD
Earn 100
Write an equation for the preparation of nitro alkanes from alkyl amines.
Important Questions on Organic Nitrogen Compounds
EASY
MEDIUM
EASY
In the reaction given below, is
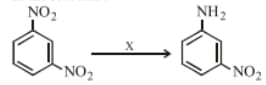
MEDIUM
HARD
Find out the product for the following reaction.
EASY
MEDIUM
HARD
A nitrogenous substance (X) is treated with and the product so formed is further treated with solution, which produces blue colouration. Which of the following can (X) be?
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
MEDIUM
EASY
Complete the given reaction.
EASY
HARD
Find out the product for the following reaction.
MEDIUM
Identify and in the given reaction.
HARD
MEDIUM
MEDIUM
Write the structures of compounds and in the following reaction.
MEDIUM
MEDIUM
EASY

