Write balanced chemical equation for — Beryllium oxide is treated separately with aqueous and aqueous solutions.
Important Questions on The s-Block Elements
Identify the ions which form hydrated chlorides from the following:
Given below are two statements:
Statement I : The chlorides of and have Cl-bridged structure. Both are soluble in organic solvents and act as Lewis bases.
Statement II: Hydroxides of and dissolve in excess alkali to give beryllate and aluminate ions. In the light of the above statements. Choose the correct answer from the options given below.
Among the statement (I – IV), the correct ones are:
(I) Be has smaller atomic radius compared to Mg.
(II) Be has higher ionization enthalpy than AI.
(III) Charge/radius ratio of Be is greater than that of Al.
(IV) Both Be and Al form mainly covalent compounds
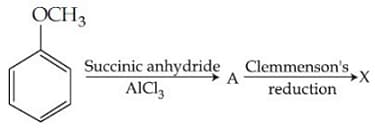
X is :
Match List with List
| List | List | ||
| (a) | (i) | Antacid | |
| (b) | (ii) | Cement | |
| (c) | (iii) | Bleach | |
| (d) | (iv) | Plaster of paris |
Choose the most appropriate answer from the
Match list-I with list-II :
| (a) | (i) | Treatment of cancer | |
| (b) | (ii) | Extraction of metals | |
| (c) | (iii) | Incendiary bombs and signals | |
| (d) | (iv) | Windows of X-ray tubes | |
| (v) | Bearings for motor engines. |
Choose the most appropriate answer, the option given below :
Reason (R) : Both and have almost same ionic radius
The correct option among the following is

