MEDIUM
9th CBSE
IMPORTANT
Earn 100
Write down the electron distribution of chlorine atom. How many electrons are there in the shell? (Atomic number of chlorine is ).

Important Questions on Structure Of The Atom
MEDIUM
9th CBSE
IMPORTANT
HARD
9th CBSE
IMPORTANT
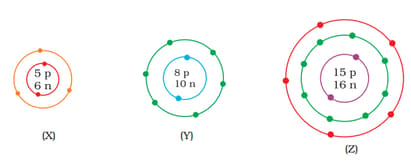
What information do you get from the figure about the atomic number, mass number and valency of atoms and ? Give your answer in a tabular form.
MEDIUM
9th CBSE
IMPORTANT
MEDIUM
9th CBSE
IMPORTANT
HARD
9th CBSE
IMPORTANT
Match the names of the Scientists given in Column A with their contributions towards the understanding of the atomic structure as given in Column B.
| Column A | Column B |
| (a)Ernest Rutherford | (i) Indivisibility of atoms |
| (b) JJ. Thomson | (ii) Stationary orbits |
| (c) Dalton | (iii) Concept of nucleus |
| (d) Niels Bohr | (iv) Discovery of electrons |
| (e) James Chadwick | (v) Atomic number |
| (f) E. Goldstein | (vi) Neutron |
| (g) Moseley | (vii) Canal rays |
MEDIUM
9th CBSE
IMPORTANT
MEDIUM
9th CBSE
IMPORTANT
Complete the Table 4.1 on the basis of information available in the symbols given below.
(a)
(b)
(c)
| Element | ||
MEDIUM
9th CBSE
IMPORTANT
Complete the Table 4.1 on the basis of information available in the symbols given below.
| Element | ` |
