HARD
JEE Advanced
IMPORTANT
Earn 100
Write down the molecular orbital diagram of (superoxide), and Find out their bond order, magnetic properties and stability.

Important Questions on Chemical Bonding and Molecular Structure
HARD
JEE Advanced
IMPORTANT
Which of the species would you expect to be stabilised by the addition of an electron to form and ionization to ?
HARD
JEE Advanced
IMPORTANT
What would be the expected electronic arrangement and magnetic moment in the superoxide ion, the peroxide ion,
HARD
JEE Advanced
IMPORTANT
Explain the observations that the bond length in is greater than in while the bond length in is less than in .
HARD
JEE Advanced
IMPORTANT
Show that if two atoms bond along their axes, the various orbitals can combine to form or (delta) type molecular orbitals.
HARD
JEE Advanced
IMPORTANT
In which pair or pairs is the stronger bond found in the first species?
:
:
:
MEDIUM
JEE Advanced
IMPORTANT
When is formed from bond-order _______ and when is formed from bond-order _______.
MEDIUM
JEE Advanced
IMPORTANT
Bond order of and is in the order:
MEDIUM
JEE Advanced
IMPORTANT
The MO electronic configuration of can be represented as follows:
Following conclusion can be drawn:
(I) It is excited state electronic configuration of
(II) It is more stable state than the ground state of molecule
III) Bond order of in excited state is one
IV) It is more likely to dissociate into two X-atoms in ground state than in excited state
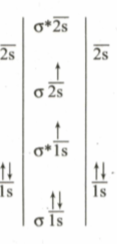
Which of the above conclusions are correct from given MO diagrams?
