Write five advantages of Electroplating.
Important Questions on Chemical Effects of Electric Current
Consider the metals and solutions given in the box.
(a) Which of the above metals have to be selected to construct a Galvanic Cell?
(b)Identify the anode and cathode of the cell.
[Reactivity order ]
(c) Write the redox reaction taking place in the cell.
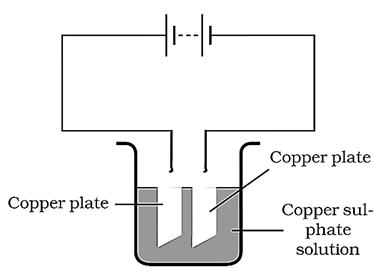
Allow the current to pass for about minutes. Now remove the electrodes from the solution and look at them carefully. Do you find any difference in any one of them? Do you find a coating over it? What colour is the coating? Note down the terminal of the battery with which this electrode is connected. The process that you saw in the above activity, is used for purification of copper. A thin plate of pure copper and a thick rod of impure copper are used as electrodes. Copper from impure rod is sought to be transferred to the thin copper plate. Which electrode should be attached to the positive terminal of the battery and why?

