Write fully balanced equations between:
(a) Copper oxide and dil. nitric acid
(b) Zinc oxide and dil. sulphuric acid.
(c) Iron (II) oxide and dil. hydrochloric acid.

Important Questions on Acids, Bases And Salts
Write fully balanced equations between:
(a) Copper hydroxide and nitric acid.
(b) Ferric hydroxide and hydrochloric acid
(c) Aluminium hydroxide and sulphuric acid.
(i) When sodium carbonate powder is treated with dilute hydrochloric acid. Write a fully balanced equation in support of your answer.
(ii) Carbon dioxide evolved in (i) above is passed through limewater for a long time. State your observations and support by two balanced chemical equations.
In the following schematic diagram for the preparation of hydrogen gas as shown in the figure what would happen if the following changes are made?
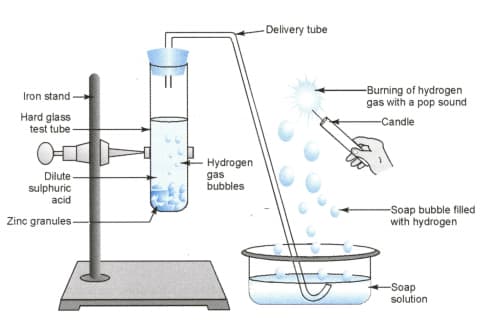
(a) In place of zinc granules, same amount of zinc dust is taken in the test tube
(b) Instead of dilute sulphuric acid, dilute hydrochloric acid is taken
(c) In place of zinc, copper turnings are taken
(d) Sodium hydroxide is taken in place of dilute sulphuric acid and the tube is heated.
And, Which of the following is acidic in nature?
(a)lime juice (b)human blood (c)lime water (d)antacid
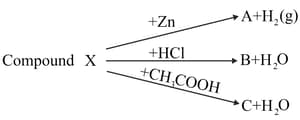
(ii)Which of the following substance will not give carbon dioxide on treatment with dilute acid?
(a)marble, (b) limestone, (c) baking soda, (d) lime.
During the preparation of hydrogen chloride gas on a humid day, the gas is usually passed through the guard tube containing calcium chloride. The role of calcium chloride taken in the guard tube is to
(i) Higher the pH, stronger the acid (ii) Higher the pH weaker the acid
(iii) Lower the pH, stronger the base (iv) Lower the pH, weaker the base
