MEDIUM
AS and A Level
IMPORTANT
Earn 100
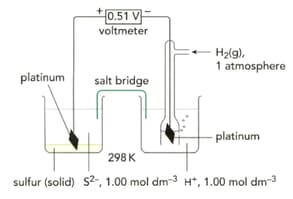
Write half-equations for the three reactions taking place in the half-cells shown on the left in the figure.
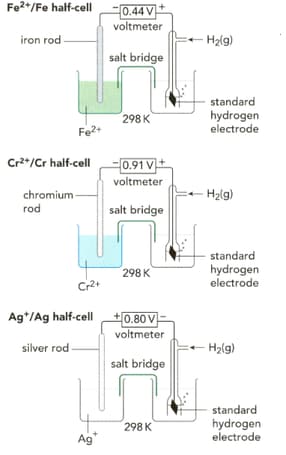


Important Questions on Electrochemistry
EASY
AS and A Level
IMPORTANT
What are the standard electrode potentials for the half-cell reactions shown on the left in the figure?
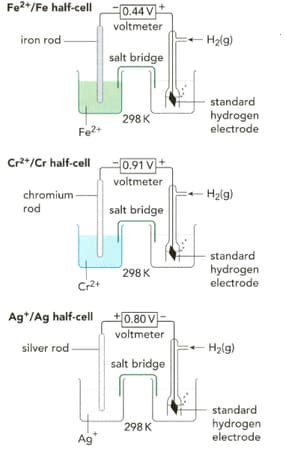
EASY
AS and A Level
IMPORTANT
List all the necessary conditions in each cell.
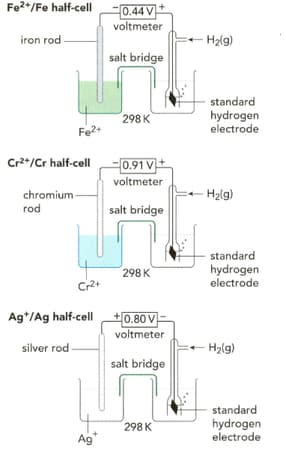
EASY
AS and A Level
IMPORTANT
Look at Figure given below. Write a half-equation for the half-cell on the left-hand side.
EASY
AS and A Level
IMPORTANT
What is the value for the half-cell given in the figure below?
MEDIUM
AS and A Level
IMPORTANT
Draw a diagram to show how you would measure the standard electrode potential for the half-cell:
Include the actual value of on your diagram.
EASY
AS and A Level
IMPORTANT
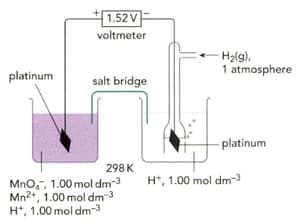
What is the value for the half-cell on the left-hand side of Figure.
EASY
AS and A Level
IMPORTANT
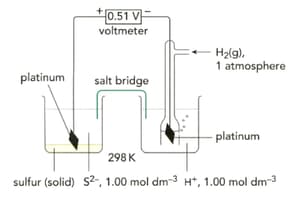
Why is platinum used in preference to other metals, in half-cells where the reaction does not involve a metallic element?
EASY
AS and A Level
IMPORTANT
Show, with the aid of a diagram, how you would measure the value for the half-cell shown by the equation:
