Write limitations of Arrhenius theory of electrolytic dissociation.
Important Questions on Electrolysis
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
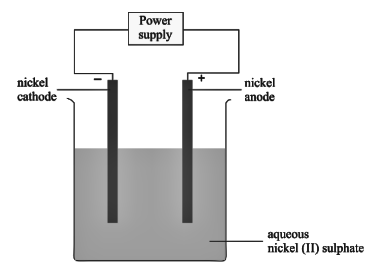
- What do you observe at the cathode and anode respectively?
(where the constant B is positive)
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
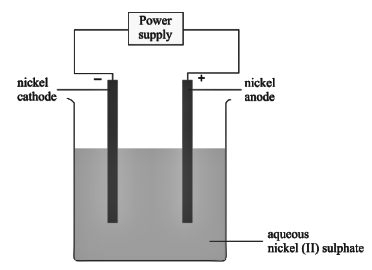
- Name the cation that remains as a spectator ion in the solution.
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
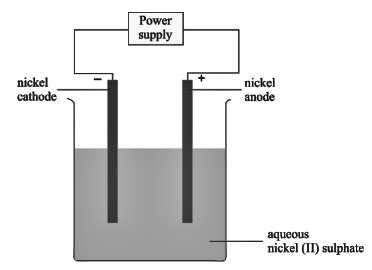
- Which equation for the reaction at the anode is correct?
Calculate the degree of dissociation of acetic acid, if its molar conductivity is .
Given: and .
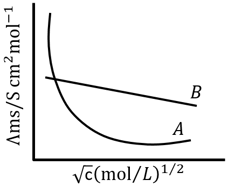
Following figure shows dependence of molar conductance of two electrolytes on concentration. is the limiting molar conductivity.
The number of Incorrect statement(s) from the following is _____
(A) for electrolyte is obtained by extrapolation
(B) For electrolyte graph is a straight line with intercept equal to
(C) At infinite dilution, the value of degree of dissociation approach zero for electrolyte .
(D) for any electrolyte or can be calculated using for individual ions.
The electronic conductivity does not depend on
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and