EASY
JEE Main/Advance
IMPORTANT
Earn 100
Write order of dipole moment of following compounds :-
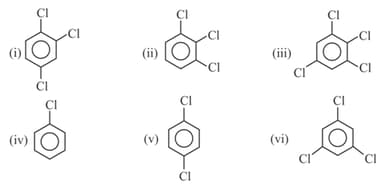

(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Chemical Bonding and Molecular Structure
MEDIUM
JEE Main/Advance
IMPORTANT
The correct order of increasing bond angle is:
HARD
JEE Main/Advance
IMPORTANT
Out of given reaction which show change in hybridisation of central atom:-
EASY
JEE Main/Advance
IMPORTANT
In the cyclo- molecule of rhombic sulphur, all the bond lengths and all the bond angles are respectively (give approximate values) :-
EASY
JEE Main/Advance
IMPORTANT
In the bond angle is but in and the bond angles are pretty close to This suggests that :-
HARD
JEE Main/Advance
IMPORTANT
Which of the following has been arranged in order of increasing covalent character?
EASY
JEE Main/Advance
IMPORTANT
contains :-
EASY
JEE Main/Advance
IMPORTANT
Rotation around the bond (between the underlined atoms) is restricted in :
EASY
JEE Main/Advance
IMPORTANT
The bond in solid can be best represented as :
