MEDIUM
Earn 100
Write the mechanism for the conversion of methyl chloride to methyl alcohol.
Important Questions on Organic Chemistry
HARD
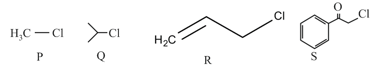
KI in acetone, undergoes SN2 reaction with each of P, Q, R and S. The rates of the reaction vary as
EASY
MEDIUM
MEDIUM
Statement II : Base causes formation of enolate anion.
MEDIUM
MEDIUM
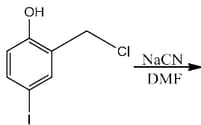
The major product of the following reaction is
HARD
EASY
EASY
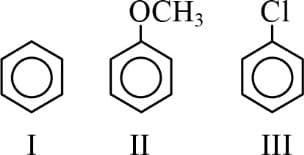
The relative reactivity towards nitronium ion is such that -
EASY
MEDIUM
HARD
MEDIUM
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and
MEDIUM
EASY
(i) Benzene
(ii) Toluene
(iii) Chlorobenzene
(iv) Phenol
EASY
HARD
EASY
EASY
(i) Benzene
(ii) Toluene
(iii) Chlorobenzene
(iv) Phenol


