Write the balanced chemical equation of any one reaction that CANNOT be classified as combination, decomposition, simple displacement or double displacement.

Important Questions on Chemical Reactions and Equations
Tina finds a paper covered with a white substance in a chemistry lab. She keeps the paper near the window of the lab and comes back to pick it up after five hours to take it home. She noticed that the white substance had turned grey.
What could be the most likely substance on the paper that Tina found?
Tina finds a paper covered with a white substance in a chemistry lab. She keeps the paper near the window of the lab and comes back to pick it up after five hours to take it home. She noticed that the white substance had turned grey.
The substance changed from white to grey. Write the chemical equation for this reaction.
Tina finds a paper covered with a white substance in a chemistry lab.She keeps the paper near the window of the lab and comes back to pick it up after five hours to take it home. She noticed that the white substance had turned grey.
State one application of this property of the substance seen in daily life.
The change in colour of moist litmus paper in the given set up is due to :
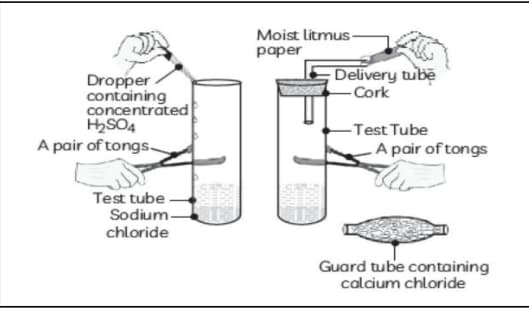
i. Presence of acid
ii. Presence of base
iii. Presence of (aq) in the solution
iv. Presence of litmus which acts as an indicator.
With the reference of above reaction which one of the option in the table is correct?
| Reactants | Products |
| (a) | |
| (b) | |
| (c) | |
| (b) |
