Write the balanced equation of sodium hydroxide with hydrochloric acid.
Important Questions on Chemical Reactions
Distinguish between the following pair of compounds using the reagent given in the bracket.
Dilute hydrochloric acid and dilute sulphuric acid. (using lead nitrate solution)
For the preparation of hydrochloric acid in the laboratory, what arrangement is done to dissolve hydrogen chloride gas in water?
Choose the method of preparation of Lead chloride, from the methods given in the list:
[List: A. Neutralization B.Precipitation C.Direct combination D.Substitution]
Manganese dioxide and copper(II) oxide. (using concentrated )
Account for the following:
When a sample of solid sodium hydroxide which has been left exposed to air for some time is added to dilute hydrochloric acid, a brisk effervescence is noted.
Action of dilute hydrochloric acid on magnesium sulphite.
The action of dilute hydrochloric acid on sodium sulphide.
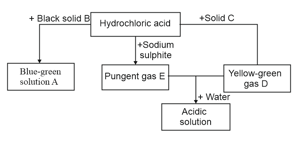
Name the products formed when aqueous sodium hydroxide is added to solution .
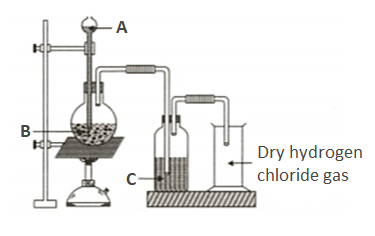
Name the substance that is added from the thistle funnel.
Lab preparation |
Reactants used | Products formed | Drying agent | Method of collection | |
|---|---|---|---|---|---|
| (i) | gas | Concentrated |
|||
| (ii) | gas |
Why is such an arrangement necessary? Give two reasons.
Hydrogen chloride is a neutral gas and dissolves in water to form an acidic solution. Explain why dry hydrogen chloride gas is neutral.
Name the gas evolved when the following mixtures are heated:
Manganese dioxide and concentrated hydrochloric acid.
Each of the gases, sulphur dioxide, hydrogen sulphide and chlorine can be obtained by the reaction of hydrochloric acid with a suitable reagent. For each of these gases, write a chemical equation for its formation by using hydrochloric acid.
Write chemical equation for the following:
Hydrogen chloride is formed from a metallic chloride.

