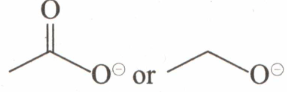Write the conjugate acid and conjugate base of the following:

Important Questions on General Organic Chemistry
Which is the stronger base in each of the following pairs?
and
Which is the stronger base in each of the following pairs?
and
Which is the stronger base in each of the following pairs?
and
Choose the member of each of the following pairs of compounds that is likely to be the stronger base.
Choose the member of each of the following pairs of compounds that is likely to be the stronger base.
Choose the member of each of the following pairs of compounds that is likely to be the stronger base.
Choose the member of each of the following pairs of compounds that is likely to be the stronger base.
or
Choose the member of each of the following pairs of compounds that is likely to be the stronger base.