HARD
10th Kerala Board
IMPORTANT
Earn 100
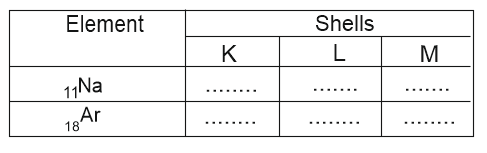
Write the electronic configuration of sodium and argon and complete the given table.


Important Questions on Periodic Table and Electronic Configuration
HARD
10th Kerala Board
IMPORTANT
MEDIUM
10th Kerala Board
IMPORTANT
MEDIUM
10th Kerala Board
IMPORTANT
EASY
10th Kerala Board
IMPORTANT
See the subshells present in each shell in the given table.
| Shell number | 1 | 2 | 3 | 4 |
| Subshells |
Which subshell is common to all shells.
MEDIUM
10th Kerala Board
IMPORTANT
MEDIUM
10th Kerala Board
IMPORTANT
Complete the given table:
| Shell number | 1 | 2 | 3 | 4 | ||||||
| Subshell | s | s | p | s | p | d | s | p | d | f |
| Representation of subshells | 1s | - | - | - | 3p | - | - | - | 4d | - |
EASY
10th Kerala Board
IMPORTANT
MEDIUM
10th Kerala Board
IMPORTANT
