EASY
Earn 100
Write the name of Thomson 's model?
Important Questions on Structure of The Atom
EASY
EASY
A beam of fast moving alpha particles were directed towards a thin film of Gold. The parts and of the transmitted and reflected beams corresponding to the incident parts and of the beam are shown in the adjoing diagram. The number of alpha particles in
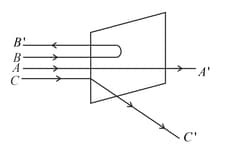
MEDIUM
MEDIUM
MEDIUM
Identify the sets of quantum numbers which are not possible?
EASY
EASY
EASY
EASY
EASY
Scattering angle
Number of scattered -particles detected
(Plots are schematic and not to scale)
MEDIUM
Match the following :
List -I :
(a) Frequency of distribution of the emitted radiation from a black body
(b) Spin quantum numbers(ms)
(c) Angular Momentum
(d) All orbital have equal energy
List - II :
(i) degeneracy
(ii) temperature dependent
(iii) vector quantity
(iv) mass times velocity times radius
Codes:
EASY
EASY
In gold foil experiment most of the -particles pass through it, it shows
MEDIUM
What should students need to learn by a specific way of working of scientists?
EASY
HARD

