MEDIUM
Earn 100
Write the reaction of addition of to ethyne.
Important Questions on Unsaturated Hydrocarbons- Alkynes
MEDIUM
Consider the following reaction:
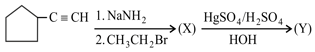
The product is
EASY
MEDIUM
HARD
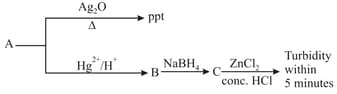
‘A’ is:
MEDIUM
The major product of the following reaction is
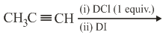
EASY
Which among the following depicts the correct order of acidity?
MEDIUM
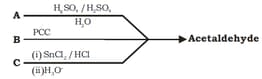
A, B and C respectively are
MEDIUM
In the compound,
The most acidic hydrogen atom is
MEDIUM
The major product in the following reactions is
MEDIUM
MEDIUM
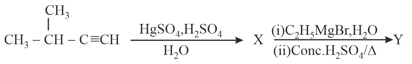
MEDIUM
In the reaction
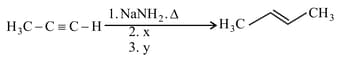
and , respectively, are
MEDIUM
The correct sequence of reagents for the following conversion will be
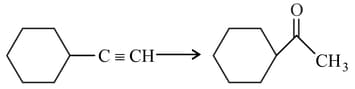
HARD
HARD
EASY
MEDIUM
HARD
EASY
MEDIUM

