EASY
Earn 100
Write the reaction of calcium oxide with carbon dioxide.
Important Questions on The s-Block Elements
EASY
Dead burnt plaster is
MEDIUM
When gypsum is heated to it forms:
EASY
Which of the reactions represents "slaking of lime"?
EASY
What is the commercial name for calcium oxide?
EASY
Match the following compounds (Column-I) with their uses (Column-II)
| S. No. | Column-I | S.No. | Column-II |
| (I) | (A) | casts of statues | |
| (II) | (B) | white wash | |
| (III) | (C) | antacid | |
| (IV) | (D) | washing soda preparation |
EASY
The suspension of slaked lime in water is known as
EASY
The substance not likely to contain CaCO3 is
EASY
Setting of plaster of Paris is
MEDIUM
The setting of plaster of paris involves:
MEDIUM
What is the difference between slaked lime and lime water?
MEDIUM
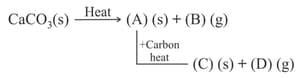
.
The compound (E)(g) is :
EASY
The compound insoluble in acetic acid is
MEDIUM
When a piece of limestone reacts with dilute , a gas is produced. When gas is passed through lime water, then a white precipitate is formed. On passing excess of gas , the white precipitate dissolves forming a soluble compound . What are and Z?
HARD
A solid substance P which is very hard is used in the construction of many buildings, especially flooring. When substance P is heated strongly, it decomposes to form another solid Q and a gas R is given out. Solid Q reacts with water with the release of a lot of heat to form a substance S. When gas R is passed into a clear solution of substance S, then a white precipitate of substance T is formed. The substance T has the same chemical composition as starting substance.
(i) What is substance P? Write its common name as well as chemical formula.
(ii) What is substance Q?
(iii) What is gas R?
(iv) What is substance S? What is its clear solution known as?
(v) What is substance T? Name any two natural forms in which substance occurs in nature.
HARD
of a substance () was treated with an excess of water. of readily combustible gas were produced along with the solution, which when reacted with gas, produced white turbidity. The substance () could be
MEDIUM
Which one of the following substances is used in the laboratory for a fast drying of neutral gases?
HARD
The correct match is/are
EASY
The chemical which is used for plastering the broken bones is
HARD
Estimation of calcium and magnesium is done by

