X and Y are elements. Two chemical species X and Y combine together to form a product P which contains both X and Y.
X and Y cannot be broken down into simpler substances by simple chemical reactions. Which of the following concerning species X, Y, and P are correct?
(i) P is a compound.
(ii) X and Y are compounds.
(iii) X and Y are elements.
(iv) P has a fixed composition.
Important Questions on Atoms and Molecules
The reaction of the burning of carbon in oxygen is represented by equation
When 9.0 g of solid carbon is burnt in 16.0 g of oxygen gas the mass of carbon dioxide gas formed would be:
(Note: Atomic mass of C-12,o u, O=16.0 u)
Select from the following figure(s) that correctly represent(s) the experimental set-up for the verification of conservation of mass in a chemical reaction.
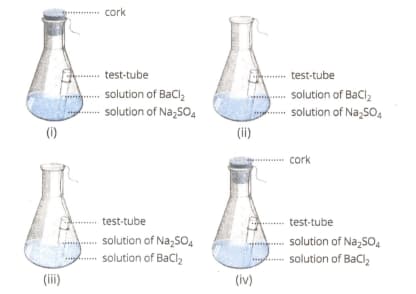
The law of constant proportion was stated by Proust as?
(a) the law of conservation of mass,
(b) the law of definite proportion?

