You are given identical balls. What is the maximum number of square voids and triangular voids (in separate arrangements) that can be created?

Important Questions on Solid State
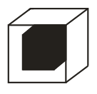
The empty space between the shaded balls and hollow balls as shown in the diagram is called:

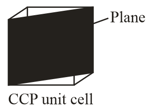
In a hypothetical solid atoms form lattice with atoms occupying all the tetrahedral voids and atoms occupying all the octahedral voids. and atoms are of the appropriate size such that there is no distortion in the lattice. Now if a plane is cut (as shown) then type of voids and their numbers which are present at the cross-section would be.
Following three planes in an unit cell are shown:
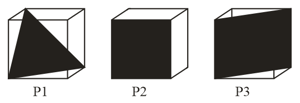
Consider the following statements and choose the correct option that follow:
(i) contains no voids of three dimensions.
(ii) contains only octahedral voids.
(iii) contains both octahedral and tetrahedral voids.
In a unit cell a cube is formed by joining the centres of all the tetrahedral voids to generate a new cube. Then the new cube would contain voids as: