You will find that electrons cannot move in definite orbits within atoms, like the planets in our solar system. To see why, let us try to observe such an orbiting electron by using a light microscope to measure the electron's presumed orbital position with a precision of, say, (a typical atom has a radius of about ). The wavelength of the light used in the microscope must then be about . What would be the photon energy of this light? How much energy would such a photon impart to an electron in a head-on collision? What do these results tell you about the possibility of viewing an atomic electron at two or more points along its presumed orbital path? (Hint: The outer electrons of atoms are bound to the atom by energies of only a few electron-volts).

Important Questions on Photons and Matter Waves
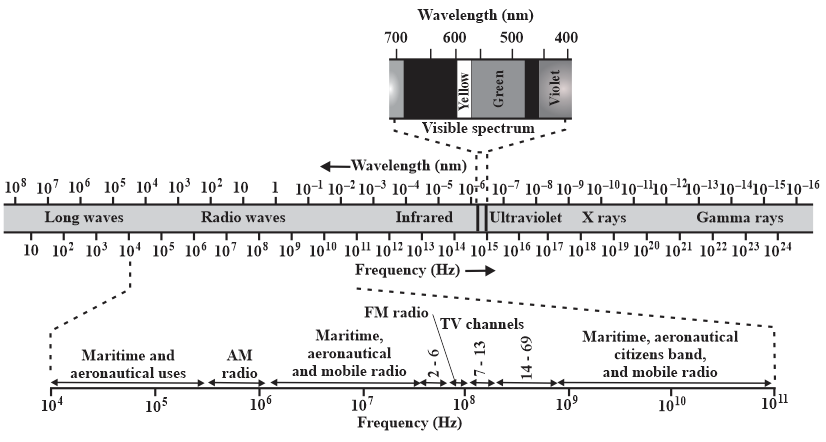
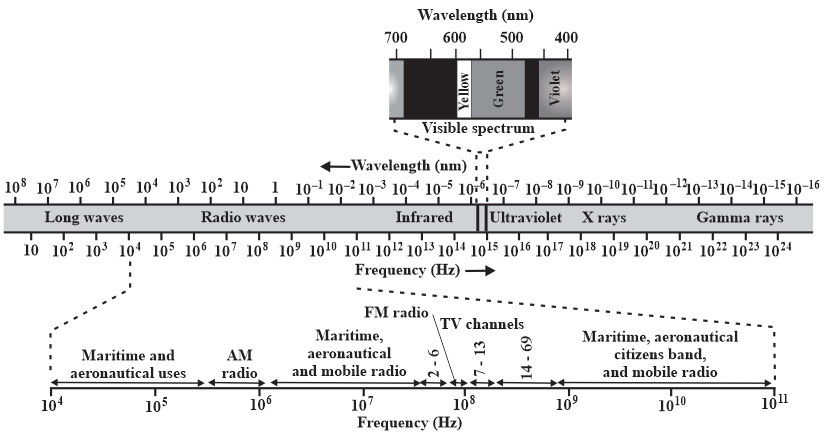
The sun is approximately an ideal blackbody radiator with a surface temperature of Find the wavelength at which its spectral radiancy is maximum and identify the type of electromagnetic wave corresponding to that wavelength. (See Figure) The universe is approximately an ideal blackbody radiator with radiation emitted when atoms first formed. Today the spectral radiancy of that radiation peaks at a wavelength of (in the microwave region). What is the corresponding temperature of the universe?
Just after detonation, the fireball in a nuclear blast is approximately an ideal blackbody radiator with a surface temperature of about .
Find the wavelength at which the thermal radiation is maximum and
identify the type of electromagnetic wave corresponding to that wavelength. (See the figure) This radiation is almost immediately absorbed by the surrounding air molecules, which produces another ideal blackbody radiator with a surface temperature of about .
Find the wavelength at which the thermal radiation is maximum and
identify the type of electromagnetic wave corresponding to that wavelength.