Zinc and iodine solution react like this:
The rate of reaction can be followed by measuring the mass of zinc metal at regular intervals, until all the iodine has been used up.
What will happen to the mass of the zinc, as the reaction proceeds?

Important Questions on The Speed of a Reaction
Zinc and iodine solution react like this:
The rate of reaction can be followed by measuring the mass of zinc metal at regular intervals, until all the iodine has been used up.
Which reactant is in excess? Explain your choice.
Zinc and iodine solution react like this:
The rate of reaction can be followed by measuring the mass of zinc metal at regular intervals, until all the iodine has been used up.
The reaction rate slows down with time. Why?
Zinc and iodine solution react like this:
The rate of reaction can be followed by measuring the mass of zinc metal at regular intervals, until all the iodine has been used up.
Sketch a graph showing the mass of zinc on the axis, and time on the axis.
Zinc and iodine solution react like this:
The rate of reaction can be followed by measuring the mass of zinc metal at regular intervals, until all the iodine has been used up.
How will the graph change if the temperature of the iodine solution is increased by ?
Zinc and iodine solution react like this:
The rate of reaction can be followed by measuring the mass of zinc metal at regular intervals, until all the iodine has been used up.
How will the graph between concentration of zinc and time changes, if the temperature of the iodine solution is increased by ? Explain your answer using the idea of collisions between particles.
Some pondweed is placed as shown:
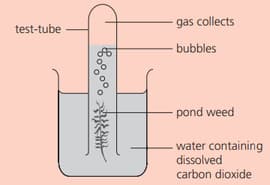
Name the gas that collects in the test tube.
Some pondweed is placed as shown:
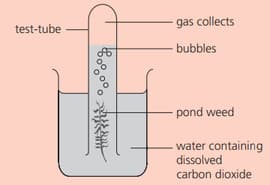
What is the products formed other than oxygen?
Some pondweed is placed as shown:
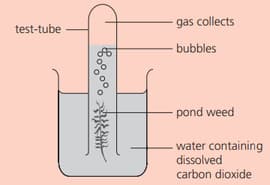
This experiment must be carried out in the light. Why?
